The recipe
Prescription of dried flowers or extracts
- In standardized quality, cannabis flowers and extracts can be prescribed and refunded.
- Can be prescribed by doctors of any specialty (excluding veterinarians and dentists).
- The prescription is based on a regular (e) prescription:
- Up to 4 e-recipes can be issued at the same time, which can be redeemed one after the other.
- Paper recipe can still be used by self-payers and in case of technical problems.
- Since 01.04.2024, they are no longer covered by the Narcotics Act.
- Only one drug may be prescribed per prescription, as this is a prescription drug.
Prescribed on:
- Pattern 16: Pink recipe: valid for 28 days
- Private recipe: Blue recipe: valid for 90 days
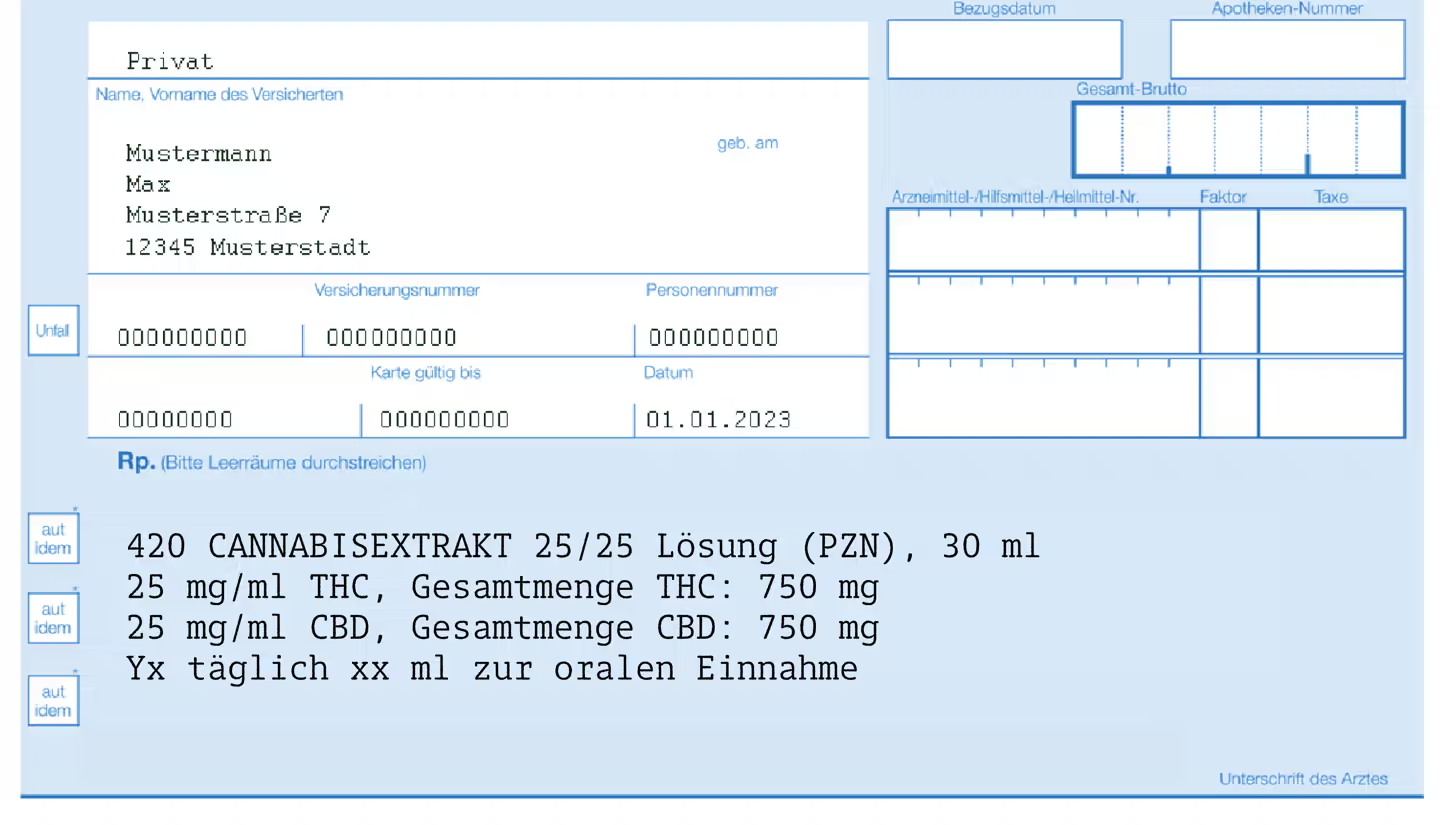
- Information from the prescriber:
- Last name, first name, job title
- Address and telephone number of the practice or clinic
- Date of issue or date of the qualified electronic signature
- Patient data:
- Name and date of birth
- Drug information:
- Name and potency
- For cannabis flowers or extracts: Information on the exact variety (including PZN for clear classification)
- If necessary: NRF manufacturing instructions or exact composition by type and quantity
- Dosage form
- Amount of drug prescribed:
- Information in grams, milliliters or the number of units divided
Substitution note: According to the “Good Substitution Practice (GSP)” for herbal medicines, such as cannabis flowers or extracts, the exchange of complex cannabinoid drugs, such as cannabis flowers or extracts, is not easily possible, even with the same THC/CBD content. If there are any uncertainties or supply bottlenecks, the pharmacy should consult the prescribing doctor. Plausibility checkCannabinoid drugs such as cannabis flowers or extracts are prescription drugs. According to § 7 ApbeTro, their prescription requires a plausibility check by the pharmacist before the medicinal product is manufactured. This comprises:
Plausibilitätsprüfung: Cannabinoidarzneimittel wie Cannabisblüten oder -extrakte sind Rezepturarzneimittel. Bei deren Verordnung ist gemäß § 7 ApBetrO eine Plausibilitätsprüfung durch den Apotheker/die Apothekerin erforderlich, bevor das Arzneimittel hergestellt wird. Diese umfasst:
- Verification of dosage, method of administration and compatibility of starting materials
- Ensuring the consistent quality and shelf life of the formulation drug
To avoid retaxations, the following points should be considered:
- Is the prescription issued correctly?
- Is there a permit from the health insurance company?
- Are the billing prices correct?
